Introduction
There are two modes of development common to most species in the animal kingdom.Virtually all embryos undergo both mosaic and regulative development at some point during their growth. Regulative development generally occurs in early gastrulation when cells are induced to form different structures according to the cell-cell signaling interactions in a specific area of the embryo that lead to the conditional specification of a cell's fate. A cell undergoing regulative development can be transplanted to another part of the embryo and form whatever structure belongs in that area instead of the structure that it would have originally formed because it is competent to receive the different signals from the new cells around it.
The second mode of development is mosaic development. Mosaic development results from the autonomous specification of a cell's fate. These cells, instead of depending on cell-cell interactions, are determined by cytoplasmic factors contained within the cell itself. These cells will form a given structure even if they are moved to a new location and are exposed to cell-cell interactions and signals that differ from their original position. Most organisms contain tissues which may undergo one or both of these developmental mechanisms at a given time (Gilbert, 2003).
The developmental pattern of the amphibian heart will be studied by bisecting the heart primordial field and observing the subsequent development of the heart, if present. If the heart were to develop using an autonomous mechanism, the cells would develop normally even if the two sides were not in physical contact with one another. This would lead to half a heart growing on either side of the incision. However, if instead the heart developed using a regulative mechanism, then the cells on the two sides would compensate for the perceived absence of the other cells. This would lead to the development of two distinct, and perhaps functional, hearts (Hamburger, 1960).
The heart morphogenetic field was split using two different techniques. First the field was split using aluminum foil. This bisection ensures that an impenetrable barrier exists between the two primordia and cuts off all signaling between cells on opposite sides of the foil. However, given that this material is completely unnatural to the embryo, this implantation technique could be excessively stressful to the embryos. Thus a second technique was modeled after the Hamburger experiment for the formation of two hearts, and involved the bisection of the heart morphogenetic field of using a transplant of non-heart tissue from older embryos. These tissues would serve as a barrier to the intracellular signals and already be specified as some other tissue. Therefore the grafts would be impervious to the heart signals. Since these transplants would be of axolotl tissue as opposed to some foreign source, such bisection would be less stressful and would presumably heal faster than bisection using foil.
Foil technique
1. Prepare microtools for surgery.
2. Prepare 1% agarose-coated operating dish; rinse with 100% HBSt and antibiotics.
3. Autoclave a small piece of aluminum foil; this foil will be used to bisect the morphogenetic fields of the heart.
4. Sort embryos by age, stop development of stage 15 axolotl embryo by placing in 4°C. The developing embryo is sensitive to temperature and will develop faster at higher temperatures (Hamburger, 1960).
5. Manually dejelly embryo in Steinbergs solution as demonstrated in video on next page. Use fine forceps to break hole in the hard jelly coat. Maneuver embryo out. Be careful not to puncture the embryo!
6. Transfer embryos into well of operating dish containing increasing concentrations of HBSt and antibiotics. Remove half the solution with pipetteman and fill with 100% HBSt. After a minute remove half the solution and fill with 100% HBSt. Allow embryo to sit 2 minutes in high salt solution. This will cause the vitelline membrane to swell and make it easier to see and remove as shown in the video. Demembranate embryo in 100% HBSt by carefully grabbing near neural crest cells with both tweezers and pulling to form a hole as described in the dejellying step above.
7. Dip all tools into 70% EtOH and then into sterile HBSt before using.
8. Hold recipient embryo ventral side up with hair loop and pick a small slit down the midline of the embryo along the anterior-posterior axis with eyebrow microscalpel or tungsten microscalpel.
CAUTION: Be gentle. Do not slice through the entire embryo and be careful not to cause inner cells to spew out of embryo!
9. Place foil in the incision deeply enough so that it does not fall out but not so deeply that it cuts through the embryo. Observe development until heart forms.
10. Leave one embryo with only the incision but no foil, and one embryo uncut as controls.
11. While the high Ca2+ concentration of the HBSt enhances healing by saturating the Ca2+ binding sites of cadherin molecules, a high salt concentration also causes developmental abnormalities. Therefore, after embryos heal from surgery, gently replace 100% HBSt with 50%, then 20% HBSt. Change solution concentrations after 12 hrs.
12. Collect pictures and movies daily using a microscope equipped with a camera and recording cameras.
Graft technique
1. Follow previous protocol for preparation of microtools and operating dishes, dejellying, and demembranation of stage 15 recipient embryos (Figure 1 A, below) and stage 20 donor embryos (Figure 1 B).
2. Position donor embryo on lateral side and recipient embryo on dorsal side (neural crest-side down) exposing ventral side in separate wells of operating dish.
3. Hold donor embryo with hair loop instrument and use Tungsten microscalpel to remove small chunk of tissue from lateral, gill-forming surface.
CAUTION: Do not remove a chunk of neural tube. These have the tendency to adhere strongly to themselves and will therefore not heal well in the recipient embryo.
4. Hold recipient embryo ventral side up with hair loop and pick a small slit down the midline of the embryo with eyebrow microscalpel or tungsten microscalpel (Figure 2 A).
CAUTION: Be gentler than when cutting the donor gill chunk. Do not slice through the entire embryo and do not cause inner cells to spew out of embryo!
5. Imbed donor gill-forming piece in the incision on the recipient embryo. Pat down and ensure its position with eyebrow or tungsten microscalpel.
6. Leave one recipient embryo with only the incision but no graft, and one embryo uncut as controls.
7. Observe the development of the embryos until heart forms.
8. As described previously, the high Ca2+ concentration of the HBSt enhances healing by saturating the Ca2+ binding sites of cadherin molecules, but a high salt concentration also causes developmental abnormalities. Therefore, after embryos heal from surgery, gently replace 100% HBSt with 50%, then 20% HBSt. Change solution concentrations after 12 hrs.
9. Collect pictures and movies daily using a microscope equipped with a still camera and recording cameras.
 need spaceB
need spaceB 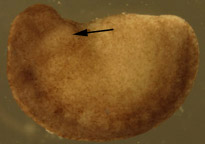
Figure 1. Dejellied test embryos. (A) Stage 15, early neurula II recipient embryo. Clear vitelline envelope is swollen and visible after soaking in 100% HBSt. (B) Dejellied, demembranated, stage 20, late neurula IV donor embryo. Donor grafts were taken from the gill-forming region of a stage 20, late neurula IV, shown by arrow.
 Need space
Need space
Figure 2. Recipient embryos. (A) Stage 16 recipient embryo with incisions down midline of ventral side. Actual incisions were larger than this photograph portrays. (B) Recipient embryo with graft in incision. Dark donor tissue sits in lighter area of incision.
Foil Procedure:
The results of the study indicate that development in the axolotl is under regulative control. Figure 1 shows a control and bisected axolotl embryos. The insicision of this particular embryo shows the bisection of the heart morphogenetic field. The movies show the result of the bisection of the heart morphogenetic field. It is clear that the bisected embryo has two hearts.


Figure 1. The bisected heart field of an axolotl embryo after three days (A). The control embryo after three days (B) Bisected embyo
Grafting Procedure:
Of the almost one hundred embryos used in the grafting procedure alone, only sixty-two survived the dejellying process. Twenty-one embryos were killed during the demembranating procedures. Twenty were in the early neurula II or III stage (stages 15 or 16) of development and appropriate for this experiment (Bordzilovskaya, 210). Eleven embryos were killed during the grafting surgery. These embryos generally had too large an incision made down the midline of the ventral side, which resulted in the spilling out of the light tan colored endo- and mesodermal cells (Figure 3). Only nine embryos were successfully grafted and set aside with the two control embryos
Forty-eight hours after surgery, most of the embryos had reached stage 27 and some of the embryos were moving by themselves (Figure 4). One embryo responded to touch by wiggling the anterior half its body back and forth. Four of the embryos had damage near the anterior end, consisting of an area where the dark ectodermal cells were missing or barely attached to the body and the lighter colored endo- and mesodermal cells were visible or hanging out the body (Figure 5A). Clumps of cells had fallen out of the embryos and were floating in the HBSt solution. After five days, seven of the nine test embryos had disintegrated into an unorganized mass of cells (Figure 5B). By day six, all embryos, including the controls, had died.

Figure 3. Surgically damaged recipient embryo. The incision was too large, resulting in the loss of light tan colored endo- and mesodermal cells. Embryos damaged in this way were ineligible for the experiment.
 Ne
Ne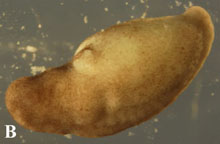
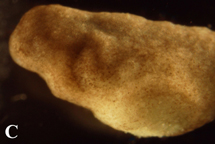
Figure 4. Post-operational development of tes
近日,服務科學領域的全球領導者賽默飛世爾科技(以下簡稱賽默飛)宣布,在達成收購意向兩個月之后,賽默飛以28億美元、折合人民幣約190億元的價格,完成了對TheBindingSiteGroup的全現金收......
11月15日,施普林格·自然和TheLens平臺宣布結成重要的合作伙伴關系,以更深入地揭示學術研究和數據如何能通過經濟和社會成效,加速推動創新的問題解決方式。通過將科學、投資和企業領域的開放數據更好地......
萬物蓬勃的7月里迎來了2022年ANTOP獎的申報和評審工作。由島津企業管理(中國)有限公司申報的“3CoinONE全新體驗氣袋進樣器”ANTOP獎進入專家評審階段。獎項名稱:3CoinONE全新體驗......
青島青源峰達太赫茲科技有限公司研發團隊在國際頂級期刊《TrendsinBiotechnology》(譯名:《生物技術趨勢》)在線發表題為“THzmedicalimaging:frominvitroto......
蛋白質作為構成人體組織器官的支架和主要物質,在人體生命活動中起著重要作用。蛋白質的相互作用能產生許多效應,如形成特異底物作用通道、生成新的結合位點、失活、作用底物專一性和動力學變化等,細胞的代謝、信號......
2021年9月9日,無錫臻和生物科技有限公司(以下簡稱“臻和科技”)與美國VyantBio公司簽署TissueofOrigin?(以下簡稱“TOO?”)全球權益和專利轉讓協議,全資收購這款唯一獲FDA......
2021年7月20日,JournalofCellularPhysiology及JournalofCellularBiochemistry同時撤回了中國學者49篇文章。從2019年開始,Journalo......
安進宣布,美國FDA授予其在研firstinclass單抗bemarituzumab突破性療法認定,與改良FOLFOX6化療方案(亞葉酸鈣、氟尿嘧啶和奧沙利鉑)聯用,一線治療FGFR2b過表達和HER......
6月10日,QS教育集團正式發布了2021年世界大學排名,中國共有83所高校上榜,包括內地高校51所,港澳臺地區高校32所。中國大學的總體排名情況已經連續數年呈上升趨勢,今年再度刷新了榜單。大學排名,......
2007年,中國科學院金屬研究所研究員張志東在英國《哲學雜志》(PhilosophicalMagazine)上發表論文,提出兩個猜想,并在猜想基礎上推定出三維伊辛模型的精確解。被《哲學雜志》審稿人評價......